profile/833629462068-66AD-4E14-8E2E-2E355A35CE2B.jpeg
Scientist

Anxiety: Why It Bothers You But Not Others
~2.9 mins read
âž¡ï¸Previous research suggests serotonin, plays a pivotal role in regulating mood and contributing to mental well-being. The brain's serotonin levels are partly controlled by proteins on the surface of brain cells - the serotonin transporter. When transporter levels are high, serotonin levels are lower, explains Shaun Quah, a neuroscience researcher at the @cambridgeuniversity & co-author of study.ðŸ§
âž¡ï¸Previously, scientists didn't exactly know how serotonin systems in particular brain regions influence individual differences in trait-anxiety.ðŸ§
âž¡ï¸Research team examined marmosets (small monkeys) & set up two experiments:-
......
🔎(1) They placed each monkey in a cage. Exposed them to a human, wearing mask, [who maintaied an eye contact with monkey for 2 mins]. They observed how the monkeys reacted before, during, and after encountering the human intruder. They tracked their avoidance level and behavioural shifts (indicative of their anxiety-level). They created anxiety scores for each animal.ðŸ§
.....
🔎The animals with the highest anxiety scores spent the majority of their time towards the back of the cage, high up, remaining relatively still, and making head and body bobs and calls, the study reports.ðŸ§
.....
🔎Researchers also analysed brain regions:- prefrontal cortex, amygdala, the dorsal anterior cingulate cortex, and raphe nuclei. They examined levels of expression for the serotonin transporter gene in these specific areas.ðŸ§
.....
🔎This revealed that monkeys with heightened reactivity (those that were the most anxious) had high levels of gene expression for serotonin transporters in their amygdala. This finding suggests serotonin signaling may be driving anxious behavior.ðŸ§
.....
📗📗2nd experiment: conducted to see if they could modulate this serotonin signaling.
âž¡ï¸Research need to be translated into humans before it can be said confidently that this version of SSRI treatment would work for people. Currently, implanting tubes specifically for anti-anxiety drug delivery into the human brain isn't a viable option, Quah says. But yes, results do suggest that targeting the amygdalae may speed up effective treatment for animals and people with trait anxiety.
🤞I hope this post is beneficial for you
Follow thebrainscifi on Instagram for more content like this.
🔎(1) They placed each monkey in a cage. Exposed them to a human, wearing mask, [who maintaied an eye contact with monkey for 2 mins]. They observed how the monkeys reacted before, during, and after encountering the human intruder. They tracked their avoidance level and behavioural shifts (indicative of their anxiety-level). They created anxiety scores for each animal.ðŸ§
.....
🔎The animals with the highest anxiety scores spent the majority of their time towards the back of the cage, high up, remaining relatively still, and making head and body bobs and calls, the study reports.ðŸ§
.....
🔎Researchers also analysed brain regions:- prefrontal cortex, amygdala, the dorsal anterior cingulate cortex, and raphe nuclei. They examined levels of expression for the serotonin transporter gene in these specific areas.ðŸ§
.....
🔎This revealed that monkeys with heightened reactivity (those that were the most anxious) had high levels of gene expression for serotonin transporters in their amygdala. This finding suggests serotonin signaling may be driving anxious behavior.ðŸ§
.....
📗📗2nd experiment: conducted to see if they could modulate this serotonin signaling.
âž¡ï¸Second experiment:- Researches selected 6 monkeys those exhibited trait-anxiety. Then, implanted thin metal tubes directly into their brains while they were under anesthesia. The team subsequently directly infused SSRI medication to the anxious monkeys' amygdalae.
.....
âž¡ï¸Researchers then repeated first experiment:- placing each monkey into the cage & exposed them to an unkown human, wearing mask, who maintained an eye contact for 2 mins & tracked monkeys' reactions (individually).
.....
âž¡ï¸After the direct infusion, monkeys experienced immediate symptom relief and expressed reduced levels of anxiety-related behaviors. Directly infusing SSRIs to the amygdalae caused a much faster anti-anxiety effect in the monkeys than typically seen with oral SSRI's medications [relief normally takes several weeks to appear,if taken orally]. .....
.....
âž¡ï¸Researchers then repeated first experiment:- placing each monkey into the cage & exposed them to an unkown human, wearing mask, who maintained an eye contact for 2 mins & tracked monkeys' reactions (individually).
.....
âž¡ï¸After the direct infusion, monkeys experienced immediate symptom relief and expressed reduced levels of anxiety-related behaviors. Directly infusing SSRIs to the amygdalae caused a much faster anti-anxiety effect in the monkeys than typically seen with oral SSRI's medications [relief normally takes several weeks to appear,if taken orally]. .....
âž¡ï¸Research need to be translated into humans before it can be said confidently that this version of SSRI treatment would work for people. Currently, implanting tubes specifically for anti-anxiety drug delivery into the human brain isn't a viable option, Quah says. But yes, results do suggest that targeting the amygdalae may speed up effective treatment for animals and people with trait anxiety.
🤞I hope this post is beneficial for you
Follow thebrainscifi on Instagram for more content like this.
profile/833629462068-66AD-4E14-8E2E-2E355A35CE2B.jpeg
Scientist
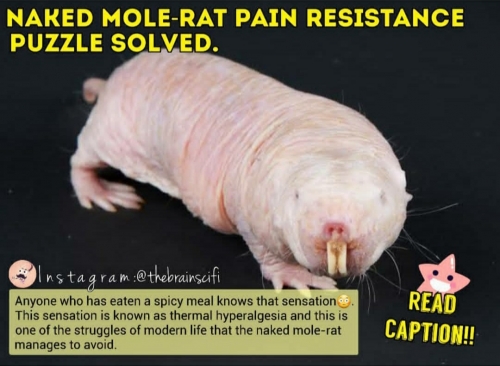
Learn Why This Rat Can't Feel Pain Ever
~1.4 mins read
If you check naked-mole rats (also called sand puppy) on wikipedia , you'll find them really fascinating. Long living and cancer resistant, with incredibly low metabolic rates. [It is the only mammalian thermoconformer, almost entirely ectothermic (cold-blooded) in how it regulates body temperature].
...
â– A 2003 study discovered their extraordinary resistance to pain. A New study published in CELL REPORTS might have worked out why.
...
âž¡ï¸Thermal hyperalgesia (TH), this response is caused when heat or inflammation causes molecules to bind around a sensory neuron receptor called TrkA, starting a signalling cascade, telling the brain to feel a burning sensation, even at normal temperatures.
Study suggests the small change in these TrkA receptors differentiates a sand puppy from other animals.
...
âž¡ï¸When naked-mole rat's TrkA compared to similar molecules in mice, study revealed a 3-amino acid change in the naked mole-rat TrkA molecule that significantly reduces it’s sensitivity, requiring roughly 10× the amount of stimulation. "Even though naked mole rat's TrkA was almost identical to that of a mouse, it has a very significant effect on the animal's ability to feel pain" says Garry R. Lewin (lead author).
...
âž¡ï¸It may even be that evolution has selected the path of TrkA to be less functional, but not to the extent of putting the animal in danger. 👉To this same end by adulthood a naked mole rat will lose around two-thirds of their pain receptors when compared to other mammals👈.
...
âž¡ï¸Why nature evolved naked mole rat this way (Although they are unlikely to encounter the capsaicin)🤔?
-They do work hard in the harsh desert climate( for food) & live underground, where close contact could make the TH reaction uncomfortably hot. Limiting this reaction allows the naked mole rats to waste less energy, making them feel more comfortable in these hot environments. 👉"They have the lowest metabolic rate of any mammal. Evolution has shut down everything that is not absolutely necessary - including extra nerve receptors," says Lewin.👈___________________
☢🤞Hope you enjoyed reading? If yes, let me know 👇 in the comments
...
â– A 2003 study discovered their extraordinary resistance to pain. A New study published in CELL REPORTS might have worked out why.
...
âž¡ï¸Thermal hyperalgesia (TH), this response is caused when heat or inflammation causes molecules to bind around a sensory neuron receptor called TrkA, starting a signalling cascade, telling the brain to feel a burning sensation, even at normal temperatures.
Study suggests the small change in these TrkA receptors differentiates a sand puppy from other animals.
...
âž¡ï¸When naked-mole rat's TrkA compared to similar molecules in mice, study revealed a 3-amino acid change in the naked mole-rat TrkA molecule that significantly reduces it’s sensitivity, requiring roughly 10× the amount of stimulation. "Even though naked mole rat's TrkA was almost identical to that of a mouse, it has a very significant effect on the animal's ability to feel pain" says Garry R. Lewin (lead author).
...
âž¡ï¸It may even be that evolution has selected the path of TrkA to be less functional, but not to the extent of putting the animal in danger. 👉To this same end by adulthood a naked mole rat will lose around two-thirds of their pain receptors when compared to other mammals👈.
...
âž¡ï¸Why nature evolved naked mole rat this way (Although they are unlikely to encounter the capsaicin)🤔?
-They do work hard in the harsh desert climate( for food) & live underground, where close contact could make the TH reaction uncomfortably hot. Limiting this reaction allows the naked mole rats to waste less energy, making them feel more comfortable in these hot environments. 👉"They have the lowest metabolic rate of any mammal. Evolution has shut down everything that is not absolutely necessary - including extra nerve receptors," says Lewin.👈___________________
☢🤞Hope you enjoyed reading? If yes, let me know 👇 in the comments
Advertisement
Link socials
Matches
Loading...
